A. Clinical trials for gene therapy of hemophilia:
Haemophilia is an X-linked disorder that severely impairs the body’s ability to make blood clots, a process that is necessary to arrest bleeding due to the insufficiency of Factor VIII (Haemophilia A) or Factor IX (Haemophilia B). If inadequately treated, the disease is associated with significant morbidity due to musculoskeletal dysfunction and early mortality due to bleeding of critical sites. Haemophilia, being a monogenic disorder is ideally suited for gene therapy through manipulation of Factor VIII or Factor IX gene to allow for prolonged and consistent expression of Factor VIII or Factor IX.
Ongoing studies with the adeno-associated virus vectors have shown good success albeit with several limitations:
(i) ineligibility of patients due to fore-existing anti-AAV antibodies,
(ii) development of an immune response to viral proteins when high doses of the viral vector were used,
(iii) partial loss of expression, particularly in factor VIII gene therapy with AAV vectors.
(iv) unexplained inter individual variation in expression.
A.1. AAV vector-based gene therapy for hemophilia B:
A unique transgene was designed for this clinical trial and packaged in the wild type AAV3 vector. This was evaluated in two in vivo models - the first by packaging this transgene into AAV8 vector and testing its functionality in the hemophilia mouse model comparing it with the LP1 promoter driven transgene (Brown et.al., Hum Gene Therapy. 2020). Gene therapy approach has inability to include very young children (<10 years old) as therapy reported in the first successful clinical trial and for the second, this transgene was packaged into AAV3 vector for evaluation in the NOG mice that carried a ‘humanized’ liver (with ~70% human hepatocytes). The first showed FIX production of about 1000ng/ml at about 3 weeks after vector infusion and in the second, this experiment also showed this transgene even when packaged in the AAV3 vector was functional expressing high levels of Factor IX expression over a period of 12 weeks in a dose-dependent manner. No unexpected toxicities were noted in both these in vivo mouse experiments. This has given us adequate data to be convinced of the functionality of this transgene cassette design and proceed towards a clinical trial. Clinical grade vector production is being organized in India for the first time.
A.2. Lentiviral vector-based gene therapy for hemophilia A:
It has been demonstrated that a hematopoietic stem cells can express Factor VIII of lentiviral vector mediated gene therapy product that has been developed in collaboration with investigators at the Emory University, Atlanta, U.S.A., for the treatment of haemophilia A. This is novel approach in the world for gene therapy of haemophilia A where the FVIII transgene is packaged in a lentiviral vector to transduce autologous hematopoietic stem cell (HSC) for stable integration and persistent transgene activity similar to the principles being applied in the gene therapy for the immune deficiency and major haemoglobin disorders. The product has been tested in pre-clinical mouse models and shown to be safe and effective. The GMP grade vector is also ready for use in a clinical trial. The principle here is to harvest mobilized HSC from the patient, ex-vivo transduce them with the lentiviral vector and then administer them back to the patient after assessment of the quality of the product and suitable conditioning of the patient as an autologous HSC transplantation. Within 3-4 weeks after transplantation production of FVIII is expected from these cells in clinically significant quantities.
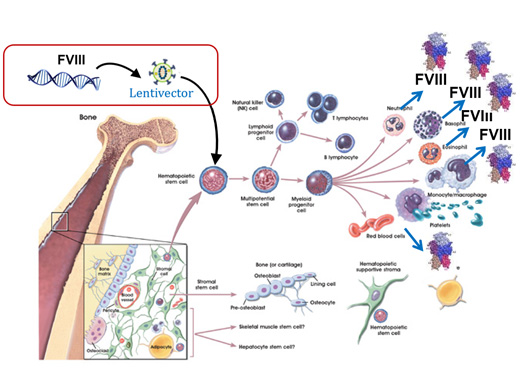
Ref: Modified image from Stem Cells: Scientific Progress and Future Research Directions. June 17, 2001; Chap-4 (The Adult Stem Cell) Pg.no. 27.
Figure 1: Lentiviral vector based FVIII gene transfer into HSCs
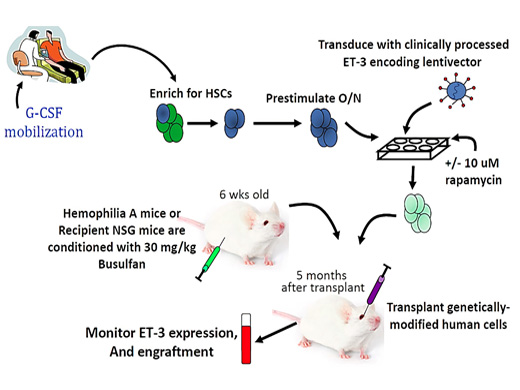
Ref: Hum Gene Ther. 2018 Oct;29(10):1183-1201
Figure 2: Phase I clinical trial of lentiviral vector-based gene therapy for haemophilia